
Assess Pharma Consultancy is an ISO 9001:2015 certified and dynamic consulting firm focused on providing various services to the pharmaceutical industries across India. Our services include Vendors and Suppliers Audits, QMS Implementation, Inspection Readiness & Remediation plans, Compliance, Gap Assessments, Trainings & Certifications, CSV & DI assessments, and technical manpower supply.
We are a group of integrity-based professionals from various technical, quality, scientific, operational, and regulatory backgrounds. We have decades of experience working in regulated pharmaceutical organizations and various multinational corporations (MNCs).
We offer innovative and practical GMP consulting services customized for the pharmaceutical industry, in line with current trends and regulatory expectations. Our services are provided by Subject Matter Experts (SMEs) with over two decades of rich experience and exposure in Pharmaceutical Quality Systems. We have partnered with consulting firms of international repute to provide need-based support.

1200
3210
3781
4300
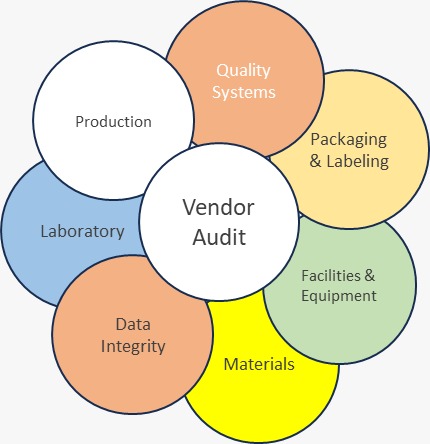
Assess Pharma Consultancy is committed to providing affordable and excellent service to our customers. We specialize in performing vendor audits for customers in the US, Europe, Latin America, Japan, Korea, and other emerging markets. Our goal is to consistently deliver high-quality vendor audit reports. Final audit reports are reviewed by subject matter experts (SMEs). Our team has extensive experience in the API industry, with our SMEs having served as heads of quality assurance (QA) in various multinational corporations (MNCs). They have also handled regulated inspections from organizations such as the US Food and Drug Administration (USFDA), the World Health Organization (WHO-Geneva), the European Directorate for the Quality of Medicines (EDQM), the Medicines and Healthcare Products Regulatory Agency (MHRA), the Ministry of Food and Drug Safety (MFDS) in Korea, the National Health Surveillance Agency (ANVISA) in Brazil, Infarmed in Portugal, the Council for the Regulation of Health Products (COFEPRIS) in Mexico, the Dutch Healthcare Inspectorate, the Indian Local Drugs Control Administration(DCA), the Central Drugs Standard Control Organization (CDSCO) in India, and ISO audits.
Assess Pharma Consultancy is committed to providing affordable and excellent service to our customers. We specialized in the implementation of Quality Management System (QMS) Our team has extensive experience in the API industry, with our SMEs having served as heads of quality assurance (QA) in various multinational corporations (MNCs). They have also handled regulated inspections from organizations such as the US Food and Drug Administration (USFDA), the World Health Organization (WHO-Geneva), the European Directorate for the Quality of Medicines (EDQM), the Medicines and Healthcare Products Regulatory Agency (MHRA), the Ministry of Food and Drug Safety (MFDS) in Korea, the National Health Surveillance Agency (ANVISA) in Brazil, Infarmed in Portugal, the Council for the Regulation of Health Products (COFEPRIS) in Mexico, the Dutch Healthcare Inspectorate, the Indian Local Drug control administration (DCA), the Central Drugs Standard Control Organization (CDSCO) in India, and ISO audits.

Creating a program that meets current regulatory expectations for cGMP/GLP compliance and prepares the firm to successfully navigate any regulatory inspection




All Rights Reserved. © 2024 Assess Pharma